What is a sporicidal? EPA, FDA, Annex I, oh my!
A Look at Regulatory Compliance for Sporicides
There are multiple guidelines in the updated Annex I specifying the use of a sporicidal agent for sterile manufacturing procedures in cleanrooms, isolators, and RABs, but what delineates a sporicidal agent from other decontamination methods? Here we take a look at the regulatory context for this word, and how to apply it in compliant standard practices.1
“The bio-decontamination process of the [isolator] interior should be automated, validated and controlled within defined cycle parameters and should include a sporicidal agent in a suitable form (e.g., gaseous or vaporized form).”
-Annex I Section 4.22.i
In its simplest definition, sporicidal refers to a substance or agent which kills spores. While tidy, this definition is too simplistic to base regulation on. In view of the liability of non-compliance with regulations and the importance of aseptic manufacturing spaces, it becomes paramount to know that your decontamination methods are up to the task. As such, our simple definition of a sporicide leaves too much room for interpretation that could lead to challenges with federal compliance in markets such as the US, Canada, and the EU. Additionally, differences may occur in the outcome and efficacy of applied practices without the use of properly approved chemicals. Fortunately, GMP facilities are not left with the burden of determining what product to use. Regulatory guidelines give a clear pathway for product choice through the standardized testing, labeling, and subsequent federal approval of a sporicidal agent.
Historically speaking, this has not always been the case. Prior to standardization of laboratory testing to vet a product’s efficacy, the EPA found 58% of registered sterilants did not perform to the level of their company claims. In order to correct these inadequacies, standardized methods for product testing were established enabling categorization of each product according to its use and efficacy.2
Who Governs What? —EPA and FDA Disinfectant and Sterilant Oversight
In the United States, two regulatory bodies oversee disinfectants and sterilants—the Environmental Protection Agency (EPA) under the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA), and the Food and Drug Administration (FDA) under the Federal Food, Drug, and Cosmetic Act (FD&C Act). The oversight of these two agencies is similar but carries important distinctions.3,4
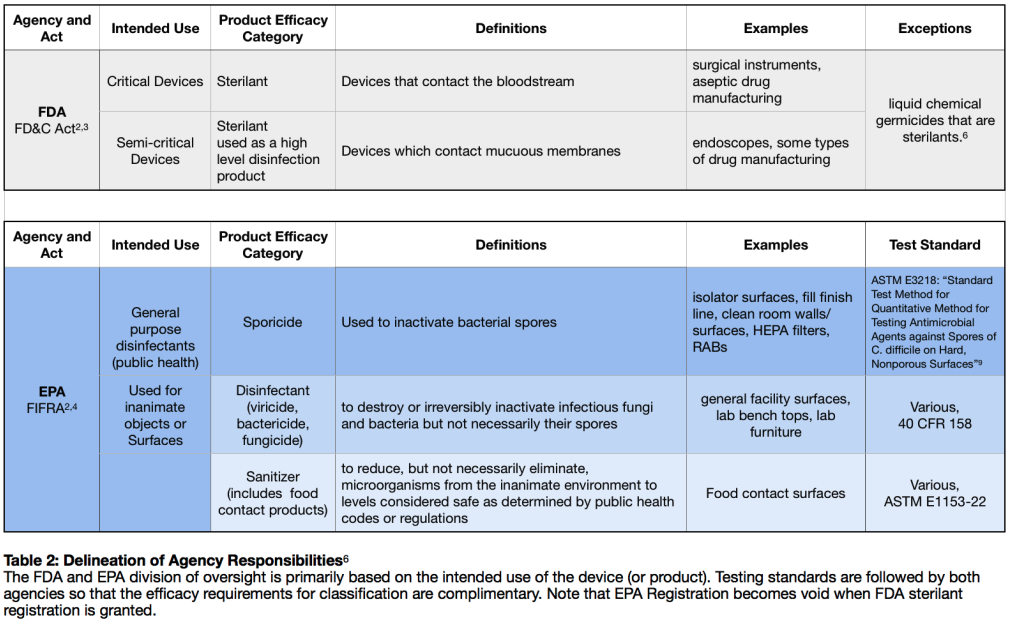
The FDA regulates ‘devices,’5 a word which they define broadly covering anything used medically in the treatment of disease or conditions of the body. By extension, the chemicals and methods used to decontaminate and sterilize these devices are considered accessories to devices and, thus, devices themselves. The FDA places devices in one of two categories—‘critical’ devices, which contact the bloodstream, and ‘semicritical’ devices which contact mucus membranes.6 FDA registration falls under Section 510(k)7 of the FD&C Act, known as a Premarket Notification, which is required both when a product is brought to market and any time significant changes are made to the product.
The EPA’s regulation under its Antimicrobials Division is the broader of the two, covering all general-purpose disinfectants and gaseous sterilants, including sporicides not used for medical devices. As a result, the EPA is the regulatory body in effect across numerous industries, from high-end pharmaceutical manufacturing and development to life science laboratories, down to even products you might use in your home.6
 It is important to note that the division of which agency oversees a registered product comes down to the intended use. The FDA is concerned with products intended for use in the body; the EPA is concerned with products intended for use for the disinfection of everything else, including all surfaces and equipment not intended for use in the body.
It is important to note that the division of which agency oversees a registered product comes down to the intended use. The FDA is concerned with products intended for use in the body; the EPA is concerned with products intended for use for the disinfection of everything else, including all surfaces and equipment not intended for use in the body.
One possible point of confusion is that the FDA regulates pharmaceutical manufacturing facilities, both by publishing standards and by performing inspections to ensure facilities are up to Good Laboratory Practice (GLP) standards. These facilities must employ sporicidal methods with either an EPA approved sporicide or possibly an FDA 510(k) sterilant product, depending on the type of manufacturing taking place at the facility and the intended use of the manufactured products. Foreseeing this potential overlap, the agencies’ efficacy standards parallel each other so that efficacy data for a 510(k) approval notice and EPA product efficacy meet each other’s equivalent standards.6
Note that EPA full approval may mirror the FDA efficacy standards but additionally requires toxicology, chemistry, ecological effects, and environmental fate data before a product can be approved. This means an EPA registered sporicide proves more than just efficacy in order to be approved. The full chemical make-up (active and inert ingredients) is studied for any potential harm to people, the facility, and the environment, and this data informs the specific safety information on the product label. Many people don’t realize that virtually all chemistries have inert ingredients. Using an EPA registered product is how you know those inert ingredients in the chemistry are within acceptable levels to be considered safe when used correctly. The same cannot be said for unregistered products.
Be Aware of Pesticide Claim Violations
No matter which governing body your product and use might fall under, it is a violation of federal law to distribute or sell a pesticide product that has not been registered with one of the two authorities or to use a registered product differently than its labeled use.
“It is unlawful for any person to distribute or sell a pesticide in the United States making claims that it will kill a particular pathogen [microorganism], unless that pesticide is registered with EPA and that particular claim has been deemed acceptable by the agency.”8
“It is a violation of federal law to use this product in a manner inconsistent with its labeling.”1
Using Sporicides in GMP Manufacturing Facilities
So where does that leave the progressive facility looking to comply with Annex I and other regulations? Let's look at two possible scenarios a facility may face and how the regulations apply.
Two GMP manufacturing facilities in the US are looking for a way to decontaminate their isolators. They need their methods to be Annex 1 compliant, so each lab knows that means they need to use a sporicidal agent.
Facility A purchases an isolator with an onboard nebulizer. They purchase an unregistered 35% hydrogen peroxide and test their process using Geobacillus stearothermophilus biological indicators to show if they have killed bacterial spores.
Facility B purchases an EPA labeled sporicidal product. They carefully read the label and use this product according to the instructions.
What do the Regulations Have to Say About Each Facility?
Table 1: Regulatory Compliance Comparison
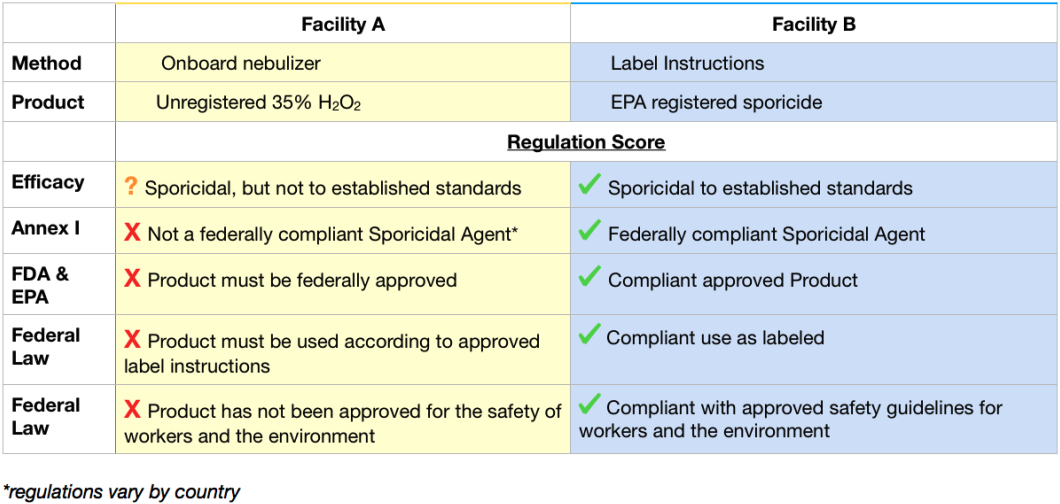
In table 1, we can see that Facility A’s decisions are not aligned with regulatory compliance. They are using bacterial spore based biological indicators, which tell them they are killing spores with their methods; however, this indicator use is not a substitution for approval from a federal regulatory body, nor is it an example of a safe chemistry. In this case, killing spores does not make this process an approved sporicide, because the process has not been tested to the accepted standards. Further, by using an unregistered product or not following the product’s instructions, Facility A is possibly in violation of federal regulations.
Conversely, Facility B chose to use an EPA registered sporicide and followed the product label’s instructions. These decisions follow regulatory guidelines, and Facility B is assured of their compliance, liability release, and peace of mind.
Steps to Ensuring Compliance with Annex I and US Regulations
Choosing a Product which:
- Is EPA registered or FDA 510(k) approved
- Is labeled as a ‘Sporicide’ or ‘Sterilant,’ depending on the intended application
- Has the equipment you intend to use the product on listed on the label; i.e, ‘for use on isolators’
- Has approval for the intended use site; i.e., ‘pharmaceutical manufacturing facility’
Consequences of Non-Compliance
Some facilities continue to use unregistered ‘off-the-shelf’ products, which may not be an EPA approved sporicidal agent or an FDA approved sterilant. Using a product which has not been required to submit efficacy data or human health and environmental impact data places the facility and manufactured product at risk. Likewise, applying any product differently than the given instructions on the label, i.e., nebulizing a liquid contact product, is not compliant with federal guidelines, because it has not been tested in the manner used. Use of non-approved disinfectants and misuse of approved products make a facility vulnerable to federal scrutiny and oversight, which can carry significant fines for misuse, mislabeling, and misleading the public. Furthermore, it is unlawful to sell or distribute an unregistered product, which can also result in hefty fines. A simple Google search will reveal the agencies’ enforcement history as well as compliance violations resulting in fines well into the million dollar range.
Bottom Line... Facilities Must Use a Federally Approved Product.
CURIS System Hybrid Hydrogen Peroxide (HHP) Technology has its own EPA registered 7% hydrogen peroxide solution and paired device to engulf a space and provide ≥6-log (99.9999%) sporicidal efficacy* and its system is approved for use as a sporicide within an isolator. The versatility and repeatability of this technology make it an ideal resource to use in spaces that need contamination control solutions with dependable sporicidal outcomes. Unlike the traditional spray and wipe method, CURIS System uses a fully automated, vaporous delivery system to help eliminate human errors. These innovative devices have onboard report generation with data tracking compliance that can be remotely operated, if desired. Using a sporicidal* system has never been easier! To learn more how CURIS System devices can meet the sporicidal needs of your facility, visit www.curissystem.com
*C-diff in a tri-part soil load
Table 2: Delineation of Agency Responsibilities
Stay Tuned...
Note that government guidelines are updated over time as industries and practices change. On February 17, 2023, the agencies released a joint Whitepaper10 which considers the roles of each agency’s oversight. As the active MOU which delineates each agency’s role is now 50 years old, both the EPA and FDA are exploring “An updated approach clarifying oversight over new and existing products” in order to “promote the efficient use of each agency’s expertise and provide clarity to the regulated industry and other stakeholders” with the goal of “creating better alignment between product regulation and the agency best equipped to regulate the product.” This update will first focus on regulations pertaining to products for animal use. We will all need to stay tuned to see what developments may follow.
For more information about the difference between general disinfectants and sporicides, visit our blog, "Evolution of Disinfection: General Disinfectants vs. EPA-Registered Sporicides."
References
- EU Commission. EU GMP Annex 1 Revision: Manufacture of Sterile Medicinal Products. Report No.: 5938. Brussels: ECA Foundation; 2022. Available from: https://health.ec.europa.eu/system/files/2022-08/20220825_gmp-an1_en_0.pdf
- Centers for Disease Control and Prevention (CDC). The Regulatory Framework for Disinfectants and Sterilants. [Internet]. Washington D.C.: U.S. Department of Health and Human Services; 2016 Sept 18. Available from: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfection-methods/regulatory-framework.html
- Food and Drug Administration (FDA). United States Code: Federal Food, Drug, and Cosmetic Act (FD&C), 21 U.S.C. §§ 301-392 (Suppl. 5 1934). [Internet] Washington D.C.: Government Printing Office; 1938 Jun 25 [Updated 2022 Dec 9]. Available from: https://www.fda.gov/regulatory-information/federal-food-drug-and-cosmetic-act-fdc-act/fdc-act-chapter-v-drugs-and-devices
- Environmental Protection Agency (EPA). Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), 7 U.S.C. §§ 136 et seq. [Internet] Washington D.C.: Government Printing Office; 1947 Jun 25 [Updated 2012 Sept 28]. Available from: https://www.epa.gov/enforcement/federal-insecticide-fungicide-and-rodenticide-act-fifra-and-federal-facilities
- Food and Drug Administration (FDA). How to Determine if Your Product is a Medical Device [Internet]. Washington D.C.: Government Printing Office; 2022 Sept 29. Available from: https://www.fda.gov/medical-devices/classify-your-medical-device/how-determine-if-your-product-medical-device
- Food and Drug Administration (FDA). Memorandum of Understanding Between The United States Food and Drug Administration Public Health Service and The Department of Health and Human Services and The United States Environmental Protection Agency. Report No.: MOU 225-93-4005. [Internet]. Washington D.C.: Government Printing Office; 1994 Jun 20. Available from: https://www.fda.gov/about-fda/domestic-mous/mou-225-93-4005
- Add: Food and Drug Administration (FDA). 510(k) Clearances [Internet]. Washington D.C.: Government Printing Office; 2021 Aug 31. Available from: https://www.fda.gov/medical-devices/device-approvals-denials-and-clearances/510k-clearances#:~:text=Section%20510(k)%20of%20the,PMN%20or%20510(k).
- Thurlow MD. Companies Entering the Disinfectant and Sanitizer Markets Should Proceed With Caution [Internet]. Atlanta, GA: BakerHostetler; 2020. Available from: https://www.bakerlaw.com/alerts/companies-entering-the-disinfectant-and-sanitizer-markets-should-proceed-with-caution
- Environmental Protection Agency (EPA). Guidance for the Efficacy Evaluation of Products with Sporicidal Claims Against Clostridium difficile. [Internet]. Washington D.C.: Government Printing Office; 2014. Available from: https://archive.epa.gov/pesticides/oppad001/web/html/cdif-guidance.html
- U.S. Environmental Protection Agency (EPA) Office of Chemical Safety and Pollution Prevention. WHITEPAPER: A Modern Approach to EPA and FDA Product Oversight. [Internet]. Washington D.C.: Government Printing Office; 2023 Feb 17. Available from: https://www.epa.gov/system/files/documents/2023-02/whitepaper-a-modern-approach-epa-fda-product-oversight.pdf