HHP™ Decontamination for Cleanrooms (LS.CLNRM)
Biodecontamination of Cleanrooms using Hybrid Hydrogen Peroxide™ Technology
Traditional cleanroom decontamination processes include multiple steps and are wrought with opportunity for potential contamination, and legacy gaseous decontamination systems, such as vaporous hydrogen peroxide (vHP) and chlorine dioxide (ClO2), employ high-consequence, high-concentration chemicals that can pose risks to staff, leave residues, or damage surfaces. To overcome many of these difficulties, CURIS tested to see if its 7% hydrogen peroxide system could provide a 6-log repeatable reduction (sporicidal disinfection) on all cleanroom surfaces as well as within filters. Request this study to see results.
Request this study
Cell Sorter Decon_Protocol for Decontamination of Becton Dickinson Cell Sorter (LS.CS)
This document provides protocol validation specific to the use of the CURIS's sporicidal disinfectant solution, a CURIS 3 HHP™ generator, and a CURIS TRINITY™ Decontamination system to decontaminate a BD FACSDiscovery S8TM Cell Sorter within a BSC. Study performed by Baker Co. in Sanford, Maine.
Request this study
Material Compatibility and Sorption of High Containment Wall and Ceiling Materials when exposed to 7% and 35% Hydrogen Peroxide Decontamination (MT.11.23)
Effects of decontamination cycles on room materials over time may cause damage that is not immediately understood to be attributable to what is being utilized for decontamination. This comprehensive material compatibility study takes a closer look at the space itself and the possible consequences repeated applications have on the integrity of building materials.
Request this study
Fill Finish Isolator Integration w/ CURIS 7000ei (LS.AST)
This case study features CURIS® groundbreaking 7000ei biodecontamination technology integrated into AST's Atmos™ fill finish isolator system for 60-minute cycle times, including aeration. Testing was performed at AST-Inc. in Tacoma, WA.
Request this study
Improve Efficiencies and Decrease Sensitive Equipment Replacement Costs with HHP Decontamination (LS.125)
Material Compatibility with Hybrid Hydrogen Peroxide on Critical Laboratory Equipment and Sensors After 125 Cycles of Exposure
Simulating real-world laboratory use, this study proves efficacy without any negative effects to sensors, lenses, or materials after repeat exposure to HHP decontamination on a particle counter's sensitive components. After 125 cycles, there were no observed residues on optical lenses or changes to materials, electronic functioning, or calibration of the particle counter, suggesting HHP as a desirable method for biodecontamination of sensitive equipment, compared to legacy systems of 35-59% vaporous hydrogen peroxide.
Request this study
HHP™ Technology for BSC Decontamination via Tenting and Taping (LS.BNG.2022)
Analysis of Use of Hybrid Hydrogen Peroxide™ Technology for Complete Biodecontamination of Biological Safety Cabinets
Laboratories, decontamination service providers, and equipment certification specialists all need to easily decontaminate biological safety cabinets (BSC). CURIS System partnered with three well-known manufacturers of BSCs (The Baker Co., Germfree, and NuAire) to determine best practices in decontaminating their particular BSCs. Multiple tests and various set ups, including tenting and taping scenarios, proved CURIS extremely efficacious in fully decontaminating all makes and models tested.
Request this study
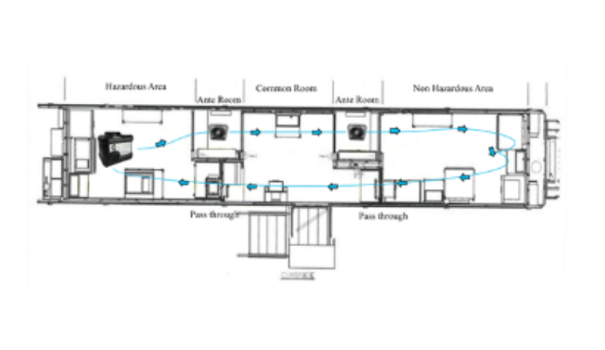
HHP™ Technology for Decontamination of a Class III BSC: Isolator / Glovebox (LS.ISGB.VT)
Efficacy of Hybrid Hydrogen Peroxide™ Fogging within a Class III Isolator
Class III biological safety cabinets, often known as isolators or gloveboxes, are used to provide sterile environments for animal research. Between projects, the interior of these devices must be decontaminated to prevent cross contamination from previous experiments. For this study, CURIS System collaborated with a university biomedical sciences and pathobiology laboratory to evaluate the CURIS TRINITY's ability to effectively decontaminate their system.
Request this study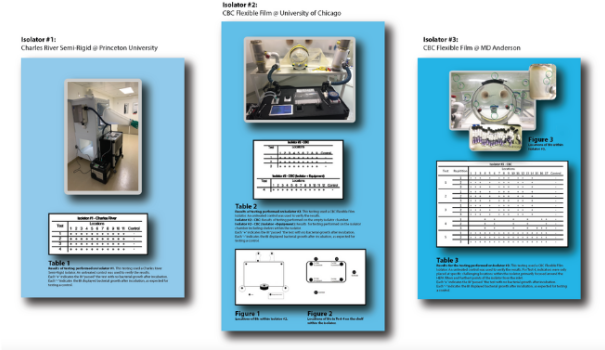
Fast, Effective Decontamination of Soft, Semi-Rigid, and Rigid Isolators (LS.CRPUUCMDA.2021)
Multi-Facility Examination of Novel Technology for Gnotobiotic Decontamination: Efficacy with Semi-Rigid and Soft-Sided Isolators
Challenges in creating a "germ-free" environment for gnotobiotic chambers, isolators, and enclosures are well known and documented. CURIS sought to innovate a system that would maintain efficacy but increase the safety, ease, and speed of gnotobiotic decontamination to greatly enhance research capabilities. In this study, researchers at MD Anderson in Texas, University of Chicago, Illinois, and Princeton University, New Jersey, proved the ability of the TRINITY device to quickly decontaminate soft-sided, semi-rigid, and rigid chambers and their filters.
Request this study
Effective IVC Rack Decontamination using Hybrid Hydrogen Peroxide in a Retrofit Chamber (ALS.IVC.2023)
Biodecontamination of an IVC Rack Using Automated, Integrated HHP™ (Hybrid Hydrogen Peroxide) Decontamination
This study completed at the University of Houston, Texas, shows the comprehensive 6-log biodecontamination of an IVC rack within a retrofit decontamination chamber using automated, integrated HHP technology, resulting in a repeatable, validatable kill throughout the animal rack and cages.
Request this study
"Hybrid Hydrogen Peroxide Technology for Viral Disinfection" in Book: 'Disinfection of Viruses' (LS.2021)
Hybrid Hydrogen Peroxide Technology for Viral Disinfection | Chapter in 'Disinfection of Viruses'
We are proud to have been invited to contribute to the book titled: Disinfection of Viruses, an essential look at methods for disinfecting against viruses, especially in the laboratory environment. Read our chapter, Hybrid Hydrogen Peroxide Technology for Viral Disinfection, for in-depth understanding of HHP™ use and efficacy, as well as a solid overview of gaseous disinfection in general.
Request this study
Efficacy in Large Laboratory and Interstitial Spaces (LS.BSL3)
Analysis of Range and Use of a Hybrid Hydrogen Peroxide System for Biosafety Level 3 and Animal Biosafety Level 3 Agriculture Laboratory Decontamination
The Biosecurity Research Institute team at Kansas State University sought to evaluate the CURIS System within their biosafety level 3 (BSL-3) and animal biosafety level 3 agriculture facility (ABSL-3Ag).
Because decontamination practices are vital to these settings to reduce cross-contamination and ensure pathogen-free spaces, they must utilize reliable and validated processes for whole-space decontamination that will not result in harm to laboratory equipment.
This study demonstrated the CURIS System's capabilities within challenging environments including some previously perceived as 'insurmountable'.
Published online ahead of print at Applied Biosafety/Liebert Publications.

Decontamination of a Room, its Contents, and HEPA Filter (LS.BC)
Effectiveness of Aerosolized Hydrogen Peroxide in Simultaneous Decontamination of a Laboratory and a Biological Safety Cabinet
Simultaneous decontamination of a laboratory and a biological safety cabinet.
Until now, this type of decontamination has required at least two separate processes enlisting multiple technologies at high costs for equipment and personnel, not to mention treatment time. This study with The Baker Company in Sanford, Maine, investigated the efficacy of the CURIS System in the decontamination of a laboratory and a biological safety cabinet using just one device and one treatment.
Request this study
Confirmation of Virological Suitability: Decon of Porous and Non Porous Materials, including N95 Masks (HC.N95DeconPS)
Aerosolized Hydrogen Peroxide Decontamination of N95 Respirators, with Fit-Testing and Viral Inactivation, Demonstrates Feasibility for Re-Use During the COVID-19 Pandemic
Penn State University in Pennsylvania sought ways to safely decontaminate N95 respirators for reuse in response to the COVID-19 pandemic. They thoroughly tested CURIS System's ability to decontaminate against three (3) viruses including SARS-CoV-2, Herpes Simplex Virus 1 (HSV-1), and Coxsackie Virus B3 (CVB3), and two (2) bacteria, including Pseudomonas and Geobacillus stearothermophilus. Without fail, CURIS' easy-to-use system and protocols successfully killed all tested pathogens. Additionally, it was proven that all treated respirators remained fully functional.
This peer reviewed study is now published on mSphere/American Society for Microbiology.
Request this study

Examination of Residual Silver and Potential Implications for Laboratory Safety (LS.Ag.2023)
Silver nanoparticles are widely used in commercial applications across numerous industries. In a laboratory setting, previous studies have examined the interaction of nanosilver with common cleaning chemistries and documented in vivo silver accumulation and its effects in laboratory mice. This material compatibility study looked at the repeated use of a common decontamination chemistry and its impact on material surfaces, finding a particulate residue throughout the test chamber following 12 cycles totaling 380 minutes of exposure.
Request this study
Enhanced Disinfection with Hybrid Hydrogen Peroxide Fogging in a Critical Care Setting (HC.2022)
Enhanced Disinfection with Hybrid Hydrogen Peroxide Fogging in a Critical Care Setting
Featured in BMC | Infectious Diseases
This research, conducted on-site at a regional hospital in the northeast US, is now available online with open access. Focus on infection prevention is increasingly vital and in critical care units, especially in a burn unit, it is imperative and potentially life saving.
"Despite ongoing efforts to advance strategies for infection control, the challenge of preventing hospital associated infections (HAIs), particularly those caused by multi-drug resistant organisms (MDROs), persists in healthcare today. .... Patients with large burns are not only more susceptible to infection but, once infected, are more likely to shed infectious pathogens into their environment, perpetuating the contamination in burn units"
Request this study
Hydrogen Peroxide Fogging: Feasibility and CLABSI Impact in a Pediatric Hematology/Oncology Unit (HC.HPF.Oncology.2024))
Hydrogen Peroxide Fogging: Feasibility and CLABSI Impact in a Pediatric Hematology/Oncology Unit
This study was initiated to address hospital acquired infections (HAIs), including central line-associated bloodstream infections (CLABSIs), which are a major contributor to morbidity and mortality in immunocompromised patients. Additionally, there was a focus on environmental contamination and bioburden caused by residues left on surfaces from traditional cleaning chemicals.
Among the key findings: "... significantly reduced CLABSI rates in the vulnerable patient population."
Study performed in conjunction with ForTec Medical in Hudson, Ohio. Additional participants noted on the study.
We invite you to request the entire study to the specifics and additional key findings.
Request this study
Emergency Preparedness during COVID-19: One Healthcare Coalition's Dynamic Response (HC.CHAPPMHDC)
Mitigating Transmission by Maximizing Disinfection Resources In Response to the COVID-19 Pandemic: One Healthcare Coalition's Strategic Response
As healthcare scrambled to battle the Coronavirus pandemic in 2020, one healthcare coalition was prepared to meet the needs of its community. By focusing on effective and versatile disinfection, with strategic communication and resource distribution, the Region K-HCC in Georgia achieved dynamic success in mitigating the challenges of a prolific COVID-19 outbreak.
Request this study

National Institute of Health (NIH) Investigated the CURIS System Ability to Penetrate Biofilm (LS.NIH)
Effective Penetration of Dry BioFilm through Pulse Hydrogen Peroxide
Effective reduction of biofilm with Pulse Hybrid-Hydrogen Peroxide: Biofilm is a hot topic in the decontamination world for good reason. Even chemicals that can kill germs often have a hard time penetrating through contaminants to get to the target pathogens. Skin oils, chemical residues, detritus, grime, and other bodily fluids layer onto surfaces, making a protective cover and/or reservoir for dangerous germs.
In this study done with the U.S. National Institutes of Health (NIH) in Bethesda, MD, CURIS System proves its ability to substantially reduce active cultures even in the presence of biofilm.
*Effective in wet and dry biofilm.
Request this study
Surgical Site Infection Reduction in the OR through CURIS Hydrogen Peroxide Fogging (HC.SSI)
Surgical Site Infection Reduction in the Operating Room through Hydrogen Peroxide Fogging
This study was initiated to address the increasing rates of hospital acquired infections (HAI’s) and surgical site infections (SSI’s) which are known to be a major contributor to healthcare morbidity.
Study performed in conjunction with ForTec Medical in Hudson, Ohio. Additional participants noted on the study.
Among the results was a notable and significant decrease in SSI rates; from 3.6% to 0.41%.
We invite you to request the entire study to learn the specifics and additional findings.

EMS Disinfection of Ambulances Using CURIS System (HC.1020)
Effectiveness of Pulse Technology and a Hybrid Hydrogen Peroxide Decontamination System for EMS
Since emergency response personnel never know the full extent of potential pathogens they may encounter, creating an environment free of biological contaminates is crucial as a staging point and finish to patient care in order to help protect them and their next patient. The Children’s Healthcare of Atlanta hospital system and Dougherty Country EMS in Georgia, partnered with Pathogend of Georgia, a bio-decontamination services company, to perform treatments using CURIS System and collect data in an effort to ensure maximum disinfection possible. The results of this study clearly show a significant and measurable improvement in the ambulance environment following treatment with the CURIS® 7% Hybrid Hydrogen Peroxide™ system.

Containing an Outbreak at the Ground Level: 100% Decrease of Norovirus in a 1600 Bed Assisted Living Facility (HC.NV)
Containing an Outbreak at the Ground Level: 100% Decrease of Norovirus in a 1600 Bed Assisted Living Facility
Knowing how a product will perform in a real-world scenario is vital: An Erickson Senior Living facility in New Jersey learned the efficacy of CURIS System in conjunction with a properly trained service company. This study discusses and examines the successful use of CURIS System and protocols to contain and stop an outbreak of Norovirus in a 1600-bed facility in a very short amount of time. In partnership with Pathogend of New Jersey.
Request this study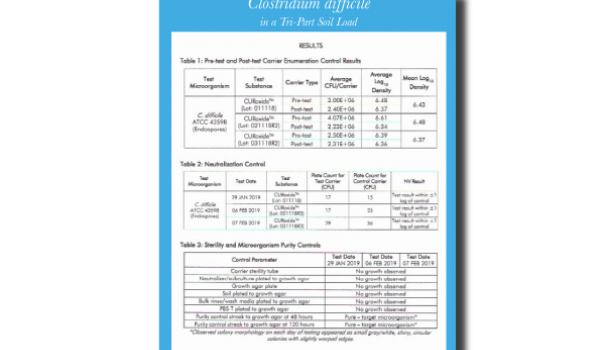
Eliminating C. diff in a Tri-Part Soil Load (EPA.REG)
Eliminating C. diff in a Tri-Part Soil Load / EPA Registered
CURIS System is proud to lead the way by meeting the EPA’s most recent and stringent C. diff efficacy requirements; the ability to kill C. diff in a tri-part soil load.
This accomplishment sets us apart from others and cements our commitment to efficacy, health, and safety.
Request this study
Feasibility and Efficacy of Daily Disinfection Practices in MASS Transit During the COVID-19 Pandemic (TRAN.2020)
Evidence of the Feasibility and Efficacy of Daily Disinfection Practices in Response to the COVID-19 Pandemic
Communities depend on public transportation for access to jobs, schools, healthcare, and basic necessities. In response to the COVID-19 pandemic, transportation providers needed to improve disinfection efforts in order to protect the health of staff and riders alike. This study looks at the feasibility of implementing large-scale, daily disinfection of each bus in a fleet, the impact on the fleet, effectiveness, sustainability, and methods employed for validation, preventing staff exposure to pathogens or chemicals. Using CURIS System and specific protocols, a mass transit organization in the State of Oregon successfully executed over 100,000 disinfection treatments over a course of less than 7 months.
Request this study
Alleviating Bio-Burden in a Wound Care Center (HC.WCC)
Alleviating Bio-Burden Risk in a Wound Care Center
Real-world disinfection often brings greater challenges than faced in standard testing. That is why CURIS System encourages everyone to validate the efficacy of their treatments. In this study, CURIS measures pre- and post- treatment levels of bio-burden to prove its ability to protect against dangerous pathogens. By combining our superior devices with custom treatment plans, CURIS successfully enabled wound care facility managers to significantly reduce the risks of HAI in their facility.
Request this study
Registration with Health Canada
CURIS Registered with Health Canada as Sporicidal Disinfectant
In addition to U.S. EPA registration as a sporicidal disinfectant, CURIS is proud to announce its proprietary 7% hydrogen peroxide solution is registered as a disinfectant with Health Canada.
Request this study
Decon Chamber for Reprocessing Class I Medical Device Packages
Discover how CURIS partnered with Spire Integrated Solutions to develop a faster, safer decontamination solution that could decontaminate unopened packages of medical devices, as well as PPE and larger items. The result was an air-tight chamber provided with a fully integrated HHP™ decontamination system enabling facilities to consistently decontaminate unopened packages of Class I medical devices that are heat and moisture sensitive, as well as PPE, electronic equipment, and durable medical equipment.
Request this study