Hydrogen Peroxide Biodecontamination—aHP, VPHP, HHP: What is the Difference?
 In the world of high-level disinfection and biodecontamination, employing the most safe and efficacious disinfection or sterilization treatment option is paramount to protecting a facility. Hydrogen peroxide has been relied on as a less caustic and highly effective method of decontamination since the turn of the century, being used in a variety of settings including in Biopharma, Biotech, Cell Gene Therapy, Research and Laboratory environments. Today, three popular gaseous hydrogen peroxide-based decontamination methods include Hybrid Hydrogen Peroxide (HHP), vapor phase hydrogen peroxide (VPHP) and aerosolized hydrogen peroxide (aHP). All have been used to target high-consequence organisms in these facilities. However, the engineering capabilities, efficacious application, versatility, and solution give HHP a significant and modern advantage over traditional devices.
In the world of high-level disinfection and biodecontamination, employing the most safe and efficacious disinfection or sterilization treatment option is paramount to protecting a facility. Hydrogen peroxide has been relied on as a less caustic and highly effective method of decontamination since the turn of the century, being used in a variety of settings including in Biopharma, Biotech, Cell Gene Therapy, Research and Laboratory environments. Today, three popular gaseous hydrogen peroxide-based decontamination methods include Hybrid Hydrogen Peroxide (HHP), vapor phase hydrogen peroxide (VPHP) and aerosolized hydrogen peroxide (aHP). All have been used to target high-consequence organisms in these facilities. However, the engineering capabilities, efficacious application, versatility, and solution give HHP a significant and modern advantage over traditional devices.
How Does Each System Work?
Aerosolized Hydrogen Peroxide (AHP) Delivery
Aerosolized Hydrogen Peroxide decontamination systems use pressure to push hydrogen peroxide (H2O2) through an atomizer. The atomizer pushes the H2O2 through a nozzle and helps form and disperse small droplets (a mist) of H2O2 into the space. It is estimated that these droplets range in size between 5 to 30 m. These large droplets may wet surfaces with concentrations of 6, 7, 10% hydrogen peroxide. Some of these delivery systems range from simple to complicated; however, they may lack the ability to accurately measure delivered disinfectant and particle sizes, making treatment cycles hard to track and replicate. There is little, if any, peer reviewed published studies proving additional efficacy on sporicidal organisms and none in a tri-part soil load.
Vapor Phase Hydrogen Peroxide (VPHP) Delivery
Vaporous hydrogen peroxide includes a vapor delivery system which traditionally incorporates high concentrations of high consequence hydrogen peroxide between 35-59% liquid. Typically, the particle sizes of these systems are below the visible range, around vapor sizes only. Particle sizes below the visible range are likely around 0.5 to .10 microns. This limited range of particles may contribute to microclimates causing inefficiency with particle distribution in a room, especially with rooms of large sizes and varying temperatures. These potential high concentration particles may condense on surfaces at much higher concentrations than the original 35-59%. Additionally, these systems are notoriously challenging to operate due to the excessive precautions necessary to avoid accidental exposure to extremely high concentrations of vapor.
Hybrid Hydrogen Peroxide (HHP) Delivery
Hybrid Hydrogen Peroxide combines vapor and micro aerosols in its delivery system to find a perfect balance and achieve maximum efficacy at the conclusion of the treatment cycle. A key difference is HHP’s innovative patented Pulse™ technology which maintains a consistent dwell time. The Pulse delivery system replenishes any spent hydrogen peroxide during the contact time, ensuring efficacy while delivering dry treatments. Pulse™ technology also ensures even distribution with a variety of vapor and micro-aerosol particle sizes in the air during the treatment cycle at a low concentration of 7% hydrogen peroxide, enabling comprehensive sporicidal high-level disinfection on surfaces air touches. This 6-log repeatable, validatable treatment utilizing the variation in particle size and the lower 7% concentration, combined with the ability to maintain optimal dwell conditions, provides HHP a distinctive advantage over Aerosolized or Vapor only systems.
Does the Chemistry of the Solution Matter?
AHP Chemistry
Although HHP, VPHP and aHP all employ hydrogen peroxide as their active ingredient, they work differently. AHP (Aerosolized Hydrogen Peroxide) often utilizes lower concentrations of hydrogen peroxide, typically employing 6%, 7%, and 10%. Many aHP devices opt to add secondary active ingredients to enhance the decontamination treatment cycle. For example, peracetic acid, a caustic chemical, is commonly combined with aHP to provide better efficacy; however, it raises concerns about staff exposure and material compatibility. Another common additive is silver. While silver has bacteriostatic properties, it can often leave behind residues which can corrode electronics or be left behind on a surface as residue, which can interfere with sensitive products or research.
VPHP Chemistry
Hydrogen peroxide vapor in high concentrations of 35%-59% is classified as a hazardous material and requires additional shipping requirements. 35% H2O2 has been known to produce 2nd degree burns and concentrations of 50% are used in rocket fuel (CDC, 1) (rxchemicals, 1). Additionally, surfaces may be subjected to a much higher concentration on surfaces than the original starting concentration, potentially exposing surfaces to corrosive levels of hydrogen peroxide (Hultman, Pharmaceutical Engineering, 2007). When gassing with these high consequence systems, the operational parts per million may reach as high as 1500 ppm. This could be dangerous in the event of accidental exposure to staff but also pose a challenge to aeration. This higher ppm potentially increases the cycles’ aeration phase and extends the amount of time required to return a room to normal operation. Additionally, high concentrations of hydrogen peroxide in vaporous form may result in bubbling paint or material incompatibility with repeated use.
HHP Chemistry
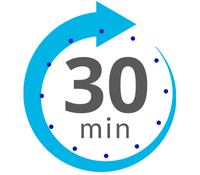
HHP™ devices utilize an Environmental Protection Agency (EPA) registered sporicidal solution with 7% hydrogen peroxide as its sole active ingredient. This H2O2 breaks down to water and oxygen, leaving behind no metal or harmful residues. Using a lower concentration of hydrogen peroxide has been associated with superior material compatibility over higher concentrations, especially on stainless steels, paint, and rubbers. Additionally, HHP’s lower concentrations typically only reach 150 ppm during the decontamination process. Although this system employs an exponentially lower hydrogen peroxide concentration, it achieves superior six-log validatable and repeatable sporicidal results. The HHP™ systems have been documented to reach 6-log efficacy and complete an entire cycle in just 30 minutes. Since no caustic chemicals are added, unlike some aHP and VPHP solutions, there are no fears of harmful properties, undesirable residues, or extremely long off gassing. It is important to remember that more chemicals or higher concentrations of chemicals added to solutions are not always better when it comes to staff safety, material compatibility, or efficacy. Since the solution is only 7%, there are no special requirements or added costs in shipping. When compared to traditional systems, CURIS HHP™ is the clear winner.
Is it possible to achieve sporicidal efficacy?
Limitations to the Effectiveness of aHP
With aHP technologies, it is difficult to tell if these systems can achieve sporicidal efficacy since there is limited peer reviewed or published data on this very important point. While some sporicidal data is available on private studies, federal approval backing their sporicidal efficacy to the latest standards has not been documented for gassing.
Effectiveness of VPHP
Traditionally, these systems produce sporicidal results and can be validated with biological indicators to prove efficacy in the decontamination envelope. This efficacy comes at a cost, since these systems have been known to peel paint off walls, pit stainless steel, and be notoriously difficult to operate.
Comprehensive Effectiveness of HHP
The HHP™ systems from CURIS System have revolutionized the gaseous decontamination market with systems capable of achieving 6-log sporicidal, repeatable results. Each cycle can be validated with biological indicators of Geobacillus stearothermophilus to prove sporicidal efficacy and has been found effective at inactivating non-enveloped viruses of 7 log, 8 log, and 10 log. This is important, since recent studies suggest achieving VPHP efficacy may be challenging with viruses.
The HHP™ systems are frequently validated with sporicidal biological indicators and have federal approval for sporicidal efficacy on the latest standards for hard to kill microorganisms, like C. diff in a tri-part soil load.
When it comes to biodecontamination, choosing a system which can produce sporicidal efficacy without the need to employ high consequence chemicals from vaporous only systems or unknown outcomes from aerosolized systems, HHP systems may be the superior choice for facilities looking to minimize their risks while maximizing their outcomes. The HHP system by CURIS System is the clear winner in all categories and a must have tool in any biodecontamination process. Whether trying to keep microorganisms out or provide contamination control, HHP systems will exceed your expectations and help your facility succeed.
Final Thoughts on how HHP™ compares to aHP and VPHP
Although aHP remains popular in non-critical environments, the increasing demand for 6-log validatable efficacy in the Life Science Laboratory environment may prove challenging for aHP success.
Legacy technologies of VPHP have traditionally ruled the Life Science space in favor of a “more means better” motto; however, recent innovations in gaseous hydrogen peroxide technologies have seen the stranglehold of the VPHP marketplace loosen significantly over the last several years due to their complicated operation and caustic properties limiting their usefulness and leaving the industry wanting for better solutions.
HHP™ technology by CURIS prevails in the Life Science decontamination sector because of its ease of use, portable design, safer* operation and repeatable 6-log efficacy. HHP™ technology has overcome the challenges that aHP and VPHP systems face and is able to compete with the efficacy of VPHP without sacrificing material compatibility or endangering staff with high concentrations of hydrogen peroxide. To learn more about implementing HHP technology in your facility, visit www.curissystem.com.
Efficacy of Hydrogen Peroxide Based Disinfection Systems
| Disinfection System | Organism Tested | Log-Reduction | Application | Pulse Injection |
| aHP | Sporicidal | 4-6log | Wet | None |
| VPHP | Sporicidal | 6-log | Dry | None |
| HHP™ (CURIS) | Sporicidal | 6-log | Dry | Yes |
*than 35-59% H2O2
Sources
Otter, J. A., Yezli, S., Barbut, F., & Perl, T. M. (2020). An overview of automated room disinfection systems: When to use them and how to choose them. Decontamination in Hospitals and Healthcare, 323–369. https://doi.org/10.1016/b978-0-08-102565-9.00015-7
IWT a tecniplast company. (2021, July). Hydrogen peroxide decontamination: Vapour and aerosol ... - tecniplast. DECON. Retrieved August 9, 2022, from https://www.tecniplast.it/usermedia/iwt/2016/brochures/WHITE_PAPER_DECON.pdf
Knobling, B., Franke, G., Klupp, E. M., Belmar Campos, C., & Knobloch, J. K. (2021). Evaluation of the Effectiveness of Two Automated Room Decontamination Devices Under Real-Life Conditions. Frontiers in public health, 9, 618263. https://doi.org/10.3389/fpubh.2021.618263
Hydrogen peroxide 50%. RXCHEMICALS. (2015). Retrieved September 7, 2022, from http://rxchemicals.com/product/hydrogen-peroxide-50
Centers for Disease Control and Prevention. (2014, October 21). Hydrogen peroxide. Centers for Disease Control and Prevention. Retrieved September 7, 2022, from https://wwwn.cdc.gov/TSP/MMG/MMGDetails.aspx?mmgid=304&toxid=55
Hultman, Carl, (Jan/Feb 2007). Physical Chemistry of Decontamination with Gaseous Hydrogen Peroxide. Pharmaceutical Engineering, 22-24.