CURIS System was formed as an answer to recurring needs throughout the decontamination industry. Traditional methods of disinfection were becoming less effective and people were becoming more chemically sensitive. To address these challenges, we focused on some basic critical components:
- Hospital-grade disinfection that can go anywhere but without sacrificing power.
- Reliably consistent and thorough treatments.
- Documentation to record and track information to determine trends to help create better practice.
- Reduced human error and cross-infection, while decreasing the use of harmful/toxic chemicals.
In response, CURIS developed the goal: To fully eliminate pathogens in virtually any space while providing a compact, all-encompassing system with a safer, easier delivery that leaves no harmful residues.
As CURIS has grown, we’ve not only addressed these initial “needs” but continued to improve on them as the world of infectious disease and infection control and prevention constantly changes. We continuously innovate and add to our already excellent line of products to make sure we meet and exceed your disinfection challenges. We are committed to making a difference in the world of decontamination that is focused on not only the response to but the prevention and control of, infectious diseases and pathogens.
Our Team
We are passionate about high-level disinfection. Everyone from our science team to our technical team to our service team brings their expertise to achieve the common goal of building a great product that achieves the highest level of efficacy, is easy to use, and can work in virtually any space.
Engineering | Design | R&D
CURIS owns its own manufacturing facility and has in-house engineering and manufacturing staff. This enables us to provide the most current designs and products to quickly meet and exceed the needs of our customers. Injection molds, 3-D printing, electronics hardware and software are a few of the in-house tools we have to continuously innovate our line of products. Software engineers, aerospace engineers, 777 airline pilots, CAD designers, and chemical and mechanical engineers make up our talented staff for Research and Development. This hardcore team of fluid dynamics experts brings its strongest designs forward to integrate into each CURIS product and bring you the most powerful possible product.
Science Team
Our Science Team is constantly adding protocols, case studies, and white papers to our already impressive Federal approvals and studies to prove CURIS' efficacy in multiple environments. This extensive scrutiny of our system ensures consistent excellence of our products for our valued customers. Whether dealing with a pandemic, a unique environmental challenge, or an outbreak, we've got you covered.
East Coast | West Coast Service Centers
We cover the needs of our customers from coast to coast with full-time staff to work on your equipment. Whether you need service for your equipment, upgrades to your devices, or installation of custom systems into your facility or vehicles, our service team has you covered.
Certified Service Providers/Trainers
Fighting an outbreak or preventing your facility from becoming a breeding ground for infection takes training, experience, and the right equipment. Our certified service providers are experts in environmental decontamination for hospitals, BSLs 2,3,&4, and educational, athletic, and corporate facilities for outbreak response and infection prevention. With backgrounds in healthcare, laboratory science, the military, emergency preparedness, remediation, and more, these service providers are trained in bio-decontamination and understand how to prevent cross-contamination and safeguard your staff and space. They use the most effective tools to provide the most comprehensive decontamination possible to leave no germ left behind.
Making a lasting difference in infection prevention and control
Germs are strengthening and becoming more resistant on a global basis to vaccines, disinfectants, and antibiotics. New pathogens, rising infection rates, and drug-resistant infections continue to be an ongoing threat.
At CURIS System, we remain focused on offering innovative solutions in portable disinfection devices to address the ever-changing threats of infectious disease.

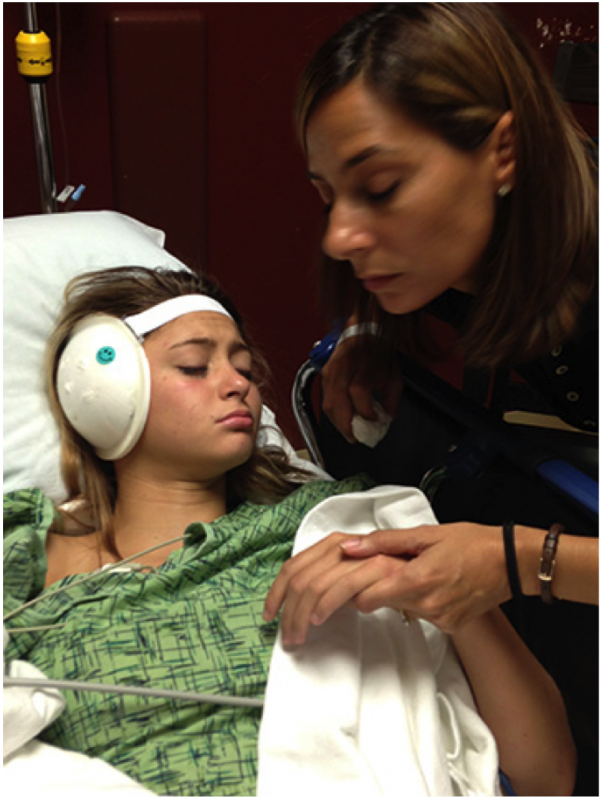
A Letter from Our Founder
A very wise gentleman once told me: “Why we do something is as important as what we do.”
Experience as preparation for what was to come
My career in the world of national and international high-level disinfection had heightened my awareness of the very real dangers of hospital-acquired infections and CAI, but it didn’t quite prepare me to personally experience the challenging and life-changing effects of fighting resistant pathogens.
Personal tragedy—community-acquired infection
As our company worked on the design and development of the CURIS fogger, my oldest daughter, Gabriella, developed a resistant staph infection in her ear after surgery for a cholesteatoma. This led to months of antibiotics, infections, and multiple surgeries, damaging her hearing and health. Throughout it all, one nagging question remained: How and where had she been exposed to this resistant staph? Finally, we narrowed it down to the weight room where she worked out each week. A community-acquired infection had just claimed another victim.
It was a devastating realization to know that she had become infected from an environmental contamination. After all, my career is centered around teaching facilities to overcome dangerous pathogens. It could have been prevented with the right disinfectants. The war against inadequate infection control just became personal.
CURIS' mission changed
We combined our original design, efficacy, and functional capabilities with systems that could be used fairly easily to help protect any facility against dangerous pathogens, outbreaks, and MDROs (multi-drug resistant organisms). Since it was apparent that infection prevention and control is not a one-and-done challenge, the system had to include remote operation and advanced data management to face these invisible threats that will be an issue for decades to come.
A happy ending
I am happy to share, that after 6 long surgeries and 6 long years of fighting, Gabriella is doing well, even though she still deals with periodic challenges related to the staph infections. Her experience has made us all more aware of the life-changing effects possible from cross-contamination or incomplete disinfection. That's why I feel we have an obligation to the facilities that trust CURIS to ensure they are well-educated in environmental decontamination.
I, more than anyone, understand that our products can be the difference between life and death. Because of this experience, I am personally obsessed with providing the most efficacious products possible. This way, anyone who uses our products can trust that we are fighting hard so that they will never have to experience what Gabriella went through or worse.
Dedicated to providing the best
I have people ask me if Gabriella's story is true. I even have competitors tell customers that her story is just a marketing gimmick—I wish it was. Unfortunately, her story is very real, but her struggle is what drives our company's passion. You can count on us to always deliver high-quality products to help you in the battle against germs. Because if you win, we all win.
Frances Grinstead
